menu
World-Class TMF Support for Biotech
eTMF Software Built to Last
Expert TMF Administration and Operational Support

We know TMF, and Startups
Our approach is designed to be robust enough for enterprise, but agile enough to for rapid implementation in a startup environment.
eTMF Software
Simple to on-board, cost-effective to manage, and packed with features that grow alongside your projects.
Full Service TMF Management
Our team not only will manage your document flow without errors, but provide you continuous feedback loops as to the quality of what's submitted.
Audit Support
Rest easy when your inspection day arrives. Our FDA audit team will be there to support all document look-ups, requests and questions.
The Challenge of Trial Master File Management
TMF Management can be expensive, cumbersome, and full of risks of mismanagement. Often the most cited with non-conformities come Audit day, your TMF left to chance can lead to headaches not only with the FDA, but future prospective acquirers.
- Massive Upfront Expense
- Often Mismanaged
- Long-term Risk
Most TMF solutions are in-flexible, and boast incredible costs to get off the ground. Our approach keeps things lean, and doesn’t penalize you for needing occasional extra help. Scale up when you need support, and down when you don’t.

Sadly TMF on multi-site studies is mismanaged more times than not, and will require costly and stressful audit/correction consultants down the line. Avoid the mess (and fees) by getting it right the first time. Our team manages daily, and performs weekly updates/audits to notify you of any growing risks in the TMF documents.
TMF organization is a common call-out during an acquisition due-diligence period. Strong and organized TMF presents a better image of your product, and ultimately fetches a higher asking price.
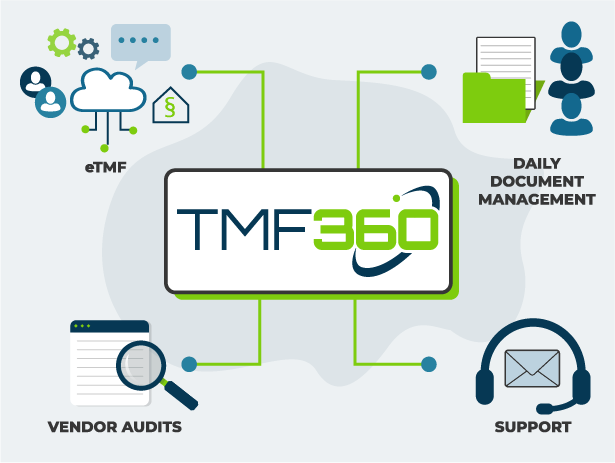
Your End-to-End TMF Solution
Technology for the Early Stage
Early-stage ventures in Biotech do not have the resources or desire to spend months and hundreds of thousands implementing software solutions that are ‘overkill’.
Our engineers focus on simplicity, and reliability. That’s why our platform is not only secure and reliable, but easily implemented and managed.
TMFPro gives you what you need, only when you need it.
Experience You Can Count On
Built-In Oversight and Support
Seasoned Pharma Veterans keep your CRO and Clinical Investigators honest. Use our checklists, and let our team maintain and manage document requests to ensure that your trials are being administered properly.
An Expert By Your Side
Come audit day, we stand by you when most firms fade into the background. Have one of our partners (with combined 50+ Years in TMF Audits) participate in your audit and respond on-site to all requests from the FDA.
Daily Management - Dedicated Support
Our team handles your TMF updates and document flow by seamlessly integrating into your trial workflow. Expect hawkish monitoring of email correspondence, and a weekly status update dashboard from your dedicated document manager.

Seed Funding Approved
The Clinical and CRO space is rife with inflated prices and underwhelming support. We are set up to deliver a higher quality service, at a price point that won’t blow a hole in your latest funding round.
Acquisition Ready Design and Implementation
TMF is not simply used for passing your audits, but when properly administered and delivered can significantly affect the valuation of your drug during acquisition due diligence.
FAQs
My CRO offered to package TMF services in with their administration of the investigations, why should we split the work to a different firm?
Two reasons, Increased quality, oversight, and budget. Ok, you got us, that was three. TMF is often lost in the shuffle when a CRO is busy running your trial and reacting to daily issues. As a customer, you often have zero insight into how things are running on the back side of the house during your trials.
Not only does our service provide far more attention and laser-like focus on your documents, we also act as a continuous auditor of the quality of documentation your CRO is producing throughout the trial.
Through our narrow focus and use of cutting edge technology, we are able to offer this superior management, service, and eTMF at a substantial discount to competitors.
